
5 Series Laser Therapy System (IR 808nm & IR 975nm Bundled Modules. Both with Combined 638nm Laser System)
Limited Time Pricing Event! (scroll for more details)
Priority Access (Pay in Full) to receive our largest discounts and priority delivery. Expected delivery in Q1 2025.
Secured RSVP ($1k Down Payment) to secure your system at a discounted price. Expected delivery in Q2 2025.
Limited Time Pricing Event!
Priority Access (Pay in Full)
- $2,000 discount on handset plus one module 808nm or 975nm
- $3,000 discount on the bundle (handset plus both modules, 808nm & 975nm)
- Priority placement in queue as part of first production batch. (Estimated Q1 2025)
- Secure your product at the lowest price available with a one-time full payment at checkout.
- Refundable* through December 31, 2024
- Taxes due upon fulfillment
- Terms & Conditions Apply
Secured RSVP (Down Payment)
- Secure your product at a discount with a $1,000 down payment
- $1,000 discount on handset plus one module 808nm or 975nm
- $1,500 discount on the bundle (handset plus both modules, 808nm & 975nm)
- Priority placement in queue as part of second production batch. (Estimated Q2 2025)
- Refundable* through March 31, 2025
- Balance + taxes due upon fulfillment
- Terms & Conditions Apply
Product Description
Designed for Clinical & Home Use
-
FDA Cleared Class II Medical Device
-
Class 3R Laser Safety Classification - protective eyewear is not required
-
Patented optical system ensures both a safe and powerful coherent-laser beam
- 528i proprietary pulsed duty cycle laser beam technology
- Supports both Continuous Wave (CW) or Pulsed laser light
-
Scratch resistant, full color LCD glass display
-
Smooth, rugged aluminum enclosure
5 Series Systems Include:
-
Laser Therapy Handset (Device)
-
Preloaded with 100+ presets optimized for indications of use
-
Compatible with all 5 Series Laser Modules
-
Bluetooth enabled for connection to mobile app
-
-
Dual Wavelength Laser Module(s) of choice
- Each wavelength can be used independently or together
-
Rechargeable and interchangeable battery with quiet-technology cooling fan
-
Mobile App Integration
-
Quick Start Guide & Digital User Manual
-
Chargeable with USB-C cables (power adapter not included)
Laser Modules included with this system:
-
Module 1
-
Infrared (IR) 808nm wavelength - 2100mW max power**
-
Red 638nm wavelength - 1500mW max power* (serves as aiming beam and emission indicator for IR therapy)
-
-
Module 2
-
Infrared (IR) 975nm wavelength - 1500mW max power**
-
Red 638nm wavelength - 1500mW max power* (serves as aiming beam and emission indicator for IR therapy)
-
* FDA Class 3R Laser Safety Hazard: Depending on power and beam area, can be momentarily hazardous when directly viewed or when staring directly at the beam with an unaided eye. Risk of injury increases when viewed with optical aids
**Pending final product validation
Pre-Orders available U.S. Only. Shipping will extend to additional countries upon regulatory approval.
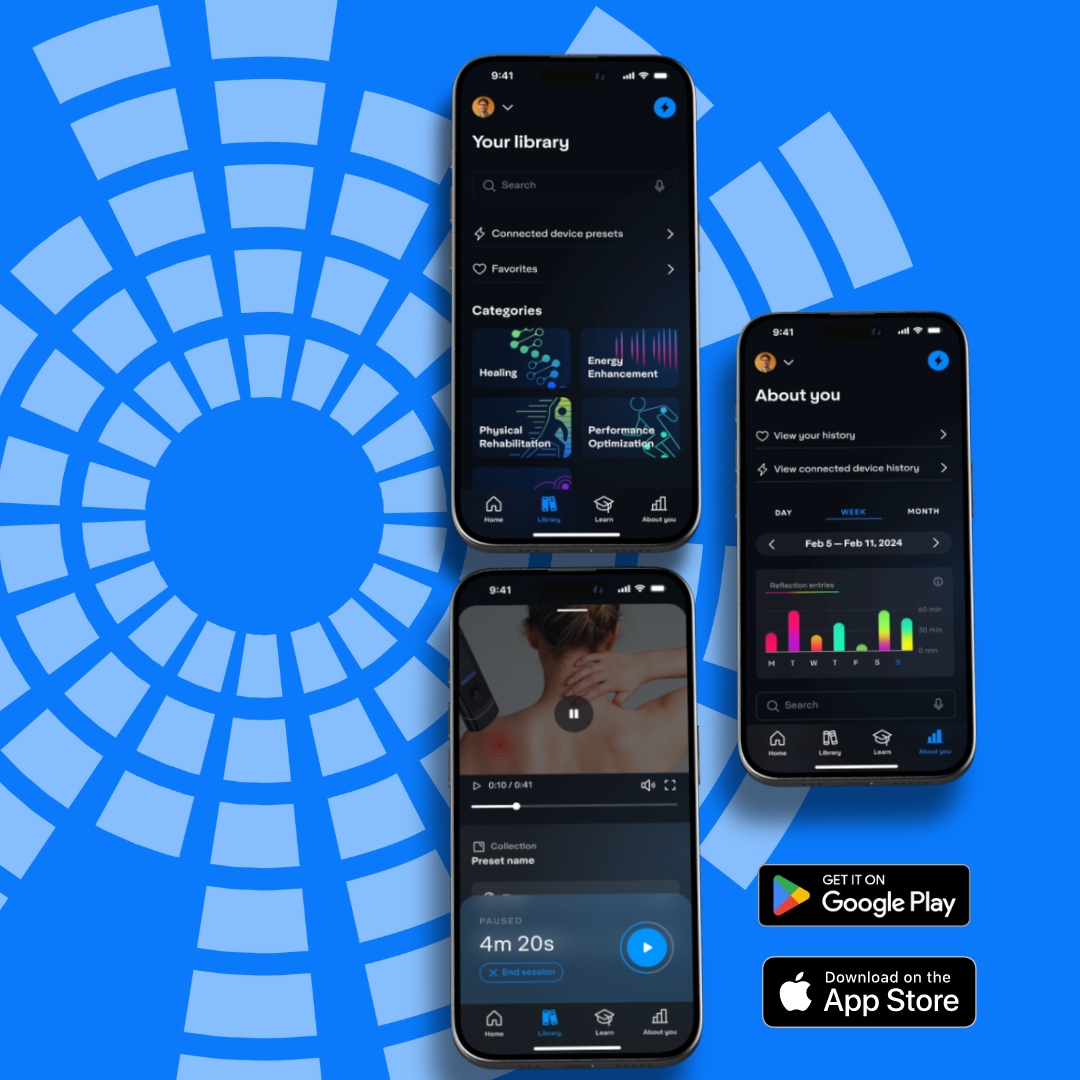
Mobile App Features
System Integration
Bluetooth connect you with your personal laser therapy system. Enable profiles for multiple users.
Guided Experience
Program specific guided videos with step by step therapy outline.
Learning Centered
Tutorials, education, videos, research & articles empower learning about therapy.
Monitor Progress
Self-monitoring, tracking of progress, usage and cataloging of favorite programs.

Experience Interactive Wellness
Coming Soon!
- Connect to wellness in NEW ways – guide for your wellness journey step by step.
- Guided Programs with optimal treatment parameters & tracking outcomes.
- Visual images act as tutorials for consistency of use & targeted treatment.
- Multiple profiles allow you to create the ultimate experience.
How to Get Started
Pre-Order Today
Ready to order?
Placing an order secures your place in line for a system, grants you access to exclusive content, and initiates your personalized experience.
Inquire
Want more information?
Fill out an inquiry form to speak with a 528i specialist who can help you select the perfect system for your needs.
Stay Connected
Don't miss out on the excitement!
Sign up for our newsletter to be the first to get updates, exclusive event access, highly anticipated products, the latest breakthroughs, and special sales.
FAQs
Is the 5-Series laser therapy system safe to use at home?
Yes. Our laser device has been intentionally engineered to meet FDA Class 3R safety classification that is considered safe when handled carefully. No safety eyewear is required to operate the laser, which is a requirement of use for Class 3b or Class 4 laser devices. At the same time, the 5-Series lasers delivers power light levels typically reserved for only clinical settings. This is a powerful laser therapy device. Never shine any laser directly into your eye, knowingly direct outwardly at someone or something, or in the air, even at lower classification levels.
What conditions can be treated?
The 5-Series laser is indicated for relief of muscle and joint pain and stiffness, arthritis pain, muscle spasms, muscle relation, and increased blood circulation. Our library of programs and presets provide optimal settings for utilization of laser therapy technology for these indications. Note: Doctors and therapists may use these therapy systems in various ways, so it is always important to understand their recommended use if professionally provided.
How long and how often should I use the device?
Therapy times vary based on condition, method of treatment, and physical characteristics of the patient (example: melanin, i.e. skin color). Therapy times can range from a few minutes to an hour. Research has shown that there are optimal dosages to maximize results, and consistent use of the same parameters provide better results over time. With other light therapy solutions, it can often be very confusing to understand how long to perform therapy. That’s why, at 528i, we provide a library of programs that personally guide you through the optimal dosing parameters and therapy times to consistently meet your wellness goals.
Are there any side effects of using a handheld laser therapy device?
Side effects are rare, but some users may experience temporary redness, warmth, or tingling in the treated area. If you experience any adverse effects, discontinue use and consult a healthcare professional.
Are there contradictions of use?
Laser therapy is considered a very safe form of therapy with little to no side effects. Never discontinue use of medications prescribed by your doctor, but report improvements. Always consult with your doctor and follow the below considerations:
- Eyes: Never direct the laser into the eyes, as it can cause serious damage or injury.
- Pregnancy: Avoid using laser therapy on the abdominal or lower back area during pregnancy.
- Cancer: Do not use the device over known or suspected cancerous lesions or tumors.
- Active Infection: Avoid using the device on areas with active infections, as the therapy might exacerbate the condition.
- Thyroid Gland: Do not use the laser over the thyroid gland to avoid potential thyroid dysfunction.
- Photosensitive Skin Conditions: Individuals with photosensitive skin conditions or those taking medications that increase photosensitivity should avoid laser therapy.
- Epilepsy: Use caution in individuals with epilepsy, especially if the device emits pulsing light that could potentially trigger seizures.
- Bleeding Disorders: Avoid using the device on areas with recent or active bleeding, as laser therapy may affect blood clotting.
Do 528i Lasers have clearance by the FDA?
528 Innovations devices are registered with the FDA and have obtained 510(k) clearance. The FDA classifies lasers based on their safety, from Class 1 (Low power output: eye exposure safe) to Class 4 (Reserve caution: strict safety protocols due to risk of eye harm). 528 Innovations revolutionized the industry with a Class 3R laser, traditionally thought to be lower in power, and through our patent pending technology is delivering power levels previously only achievable with Class 4 lasers, but without the associated hazards. Our proprietary optical system allows for high total power output in a safe manner, making it ideal for both home use and medical practices.
The FDA requires that manufacturers and distributors of medical devices in the United States register annually and submit a device list, a process known as “establishment registration.” 21 C.F.R. § 807 (2018); FDA, Device Advice: Comprehensive Regulatory Assistance: How to Study and Market Your Device: Device Registration and Listing, available at
https://www.fda.gov/medical-devices/how-study-and-market-your-device/device-registration-and-listing
The purpose of this registration requirement is to inform the FDA where medical devices are made, in the event of a public health emergency to which the agency must respond. 528 Innovations’ establishment registration can be found here: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm?rid=328500



